Difference between revisions of "Using Glass or Fused Quartz Capillaries"
| Line 27: | Line 27: | ||
[[Image:ReactivityofFusedQuartz.png|500px |link=https://wiki-ext.aps.anl.gov/ug11bm/images/6/61/ReactivityofFusedQuartz.png|alt=Alt text|Click for a larger view of ReactivityofFusedQuartz.png]] | [[Image:ReactivityofFusedQuartz.png|500px |link=https://wiki-ext.aps.anl.gov/ug11bm/images/6/61/ReactivityofFusedQuartz.png|alt=Alt text|Click for a larger view of ReactivityofFusedQuartz.png]] | ||
(credit to [http://www.momentive.com/Products/Main.aspx?id=20351 http://www.momentive.com/]] Downloaded March 10, 2014 from [http://www.momentive.com/WorkArea/DownloadAsset.aspx?id=24702] | (credit to [http://www.momentive.com/Products/Main.aspx?id=20351 http://www.momentive.com/]] | ||
Downloaded March 10, 2014 from [http://www.momentive.com/WorkArea/DownloadAsset.aspx?id=24702] | |||
== Sample Loading Video == | == Sample Loading Video == | ||
Revision as of 19:52, 10 March 2014
Capillaries: Thin Wall Glass and Fused Quartz
Thin wall glass or fused quartz capillaries are often used in powder diffraction experiments.
These are available from several vendors such as:
- Charles-Supper http://www.charles-supper.com
- Hampton Research http://hamptonresearch.com
Typical both glass ('special' or 'borosilicate') or fused (amorphous) quartz capillaries are available.
As purchased from both vendors above, the tubes are pre-sealed on one end, and have a flared opening on the other end for easy powder loading.
When selecting a capillary material and size, consider your experiment and sample(s).
Glass and quartz are stable over different temperature ranges. Glass adds a lower background to a diffraction pattern and is easier to seal with a flame (you can use Bic style lighter), but fused quartz tubes are stronger, more chemical resistant, and more likely to survive shipping and handling.
More Details
Read more details about Capillaries on the Supplies_and_Tools 11-BM Wiki page
Reactivity of Fused Quartz
In general use, fused quartz is unreactive, including when exposed to most acids, metals, chlorine and bromine at ordinary temperatures. It is slightly attacked by alkaline solutions, and the reaction rate increases with temperature and concentration of solution. Carbon and some metals will reduce fused quartz; basic oxides, carbonates, sulfates, etc., will react with it at elevated temperatures.
Click on the image below to view a useful guide of Fused Quartz Reaction With Selected Elements And Compounds At Elevated Temperatures
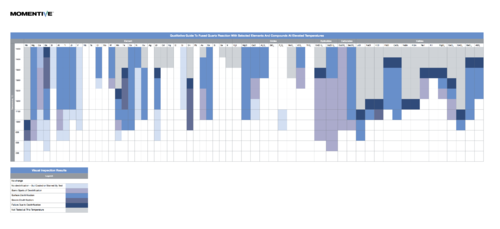 (credit to http://www.momentive.com/]
(credit to http://www.momentive.com/]
Downloaded March 10, 2014 from [1]
Sample Loading Video
Loading thin walled glass or fused quartz capillaries
Demonstration video illustrating one way to prepare standard samples for powder diffraction measurements using thin walled glass or fused quartz capillaries