Difference between revisions of "Samples with Strong X-Ray Absorption"
| Line 59: | Line 59: | ||
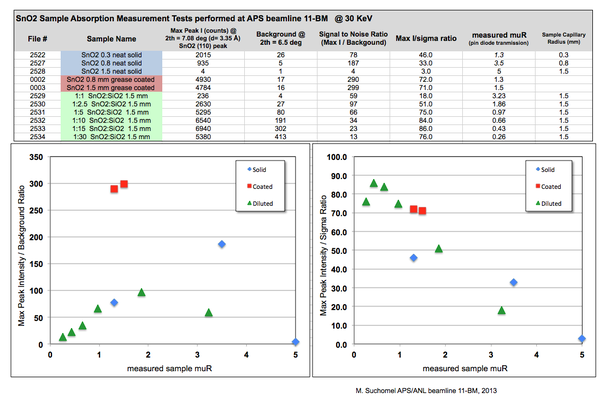
[[Image:SnO2_SpreadSheet.png| | ''Click for a larger view'' | ||
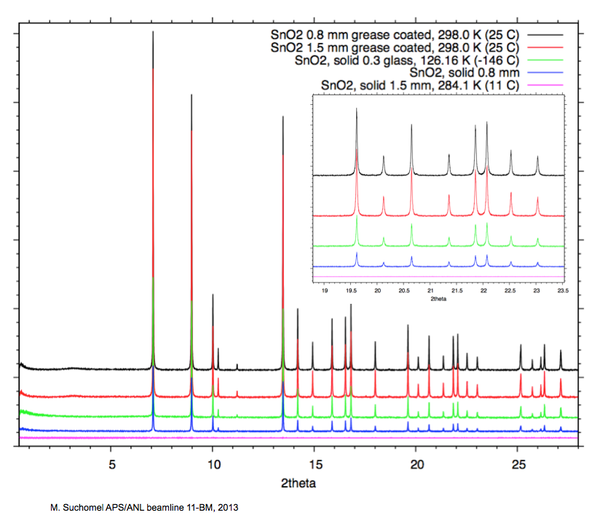
[[Image:SnO2_XRDPlot.png| | [[Image:SnO2_SpreadSheet.png|600px|SnO2_SpreadSheet.png]] | ||
[[Image:SnO2_XRDPlot.png|600px|SnO2_XRDPlot.png]] | |||
== Preparation methods to lower absorption == | == Preparation methods to lower absorption == | ||
Revision as of 00:39, 22 February 2014
Considerations for strongly absorbing samples are discussed below.
Background
A brief discussion on X-ray absorption theory can be found on the Wiki page X-ray Absorption & Fluorescence.
11-BM uses transmission (Debye-Scherrer) geometry, therefore sample x-ray absorption (μ) must be considered. Absorption is not normally a problem for most users - the high energy beam (~ 30 keV) is easily able to penetrate these samples. However, absorption can be an issue for materials containing a large fraction of high-Z elements.
A highly absorbing sample will result in a transmission powder pattern with attenuated diffraction peak intensities, especially at low 2 θ angles (the sample is absorbing both the incoming and diffracted x-rays). It may not be possible to analyze these data in any meaningful way.
For samples with moderate absorption, peak intensities are still attenuated, but it is less dependent on scattering angle. Most refinement software packages can correct for this effect (or it can be incorporated in the refined atomic thermal parameters). For samples with low absorption, this effect is negligible and can be ignored.
The total absorption of any sample can be calculated before a measurement. It is a function of the sample composition, packed sample density, capillary radius, and the x-ray wavelength. One first determines the absorption for the composition and x-ray wavelength (μ), then calculates the total absorption (μ*r) for the actual sample to be measured using the capillary radius (r), sample density and packing fraction.
Guidelines for 11-BM Mail-In Samples
For its mail-in program, 11-BM uses a wavelength of ~ 0.41 Å (30 keV), and the standard supplied capillary tubes have a radius of 0.04 cm (~0.8 mm diameter). Larger capillary tubes are now also available.
Use the convenient Absorb web utilty to estimate sample absorption: http://11bm.xor.aps.anl.gov/absorb/absorb.php
Range of μ*r values
The experimental implications for a range of sample absorption values (μ*r) are given below:
- μ*r < 0.5 : Very low absorption (might want to use a larger diameter sample to improve the signal!)
- 0.5 < μ*r < 1.5: Normal - this range is OK, for very precise thermal parameters a small correction may be needed
- 1.5 < μ*r < 2.5: High - absorption correction recommended for analysis
- μ*r > 2.5 : Absorption is too large - consider an alternative sample preparation (see below)
Practical Example
As a practical example, 11-BM staff have examined different methods of preparing powders of the x-ray absorbing oxide SnO2 for transmission capillary diffraction measurements 30 KeV on the APS beamline 11-BM.
The main results are highlighted in the images and plots below.
In theory, dilution and reduced capillary sizes should work, but in practice its not always the case.
I played with this sometime last year and made nice plots (which have only existed on my computer up to this moment). I attempted a somewhat systematic study on SnO2 powder at 30 KeV, and the main results are shown in the attached image file.
I don't bother here with estimated muR, but rather try to measure it on each sample using the pin diode after the capillary
I'd say that the message for you is that coated gives the best ratio of Intensity to background, so small peaks are better resolved; but overall signal / sigma ratio doesn't change as dramatically.
The main secret is that large diameter coated tubes use the full vertical height (~ 0.6 mm FWHM) of the xray beam, while a smaller diameter capillary with the same muR value wastes all the photons which sail above and below the tube.
Also - for dilution, you start to loose once the molar ratio goes above 5:1 (muR < 2 in this case)
Not taken into account here are funky absorption effects on relative peak intensity ratios as a function of 2th, something that one would for sure need to consider for the higher muR numbers.
Hope this helps you to think about an approach for the next samples; maybe I'll be inspired to now post this cleaned up file with the results on the webpage wiki. One day I'd hope to put the results in a short paper which can be used as a reference for the online muR calc tool.
all the best Matthew
Preparation methods to lower absorption
To decrease the μ*r of a highly absorbing sample, one must lower its effective packed density or reduce its radius.
SPECIAL NOTE January 2013: A recent series of 11-BM measurements suggests that an appropriately diluted (with glass silica etc) sample packed into a standard size capillary results in data better signal/noise than an non-diluted sample in a smaller diameter capillary with equivalent muR. Watch for more details here in the near future. Email 11-BM staff with questions in the meantime.
Dilution
One trick is dilute your sample by mixing with a lower density and amorphous material (no extra Bragg peaks!) to lower the overall absorption. Good candidates for this dilution method include silica (SiO2 glass) powder (you can grind it by hand using a mortar and pestle) or amorphous boron.
Nested Capillary
An absorbing powder sample could also be loaded into a smaller diameter capillary, which is then inserted into the standard 11-BM Kapton tube.
Be sure to record the relative amounts of sample and dilution material to determine the average composition of your mixed powder. Once the mixed powder sample is loaded in a capillary, you can determine the actual packed density by measuring the total weight (minus the empty capillary) and volume of the packed sample (packed length * π * r2).
Read our guidelines on nesting capillaries
Coated Capillary
In extreme cases for samples with very high absorption one might consider coating the *inside* of the Kapton capillary with a layer of sample powder mixed with a silicone grease. Or coating the outside of a smaller diameter nested capillary. The sample powder must be *inside* the supplied Kapton tube for mail-in samples.
Using this approach you essentially end up with a hollow tube of sample powder. This gives a good sized sample target with good powder averaging statistics, but without the absorption problems of a solid sample.
YouTube Demo Video (NEW!)
YouTube LINK - Watch video instructions on how to prepare a grease coated capillaries for 11-BM powder XRD measurements
Important !! If you plan to use a large diameter mail-in base and nest a smaller size coated Kapton tube, please send 11-BM staff an additional email directly after you request your bases and state this so we can be sure to send both size Kapton tubes. Read more here: https://wiki-ext.aps.anl.gov/ug11bm/index.php/Mail-In_Bases
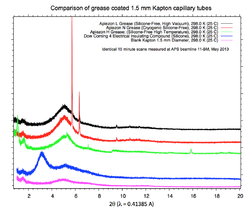
Careful! Do not use that random grease you find in the lab without first checking it on the lab XRD. Dow Corning #4 Electrical Insulating Compound silicone grease is recommended. (see plots below). Other silicone greases may also work. Less ideal choices include Vaseline and other high vacuum greases. These greases contain an crystalline phase which will add small peaks to the sample diffraction pattern. See an example of this plotted here for different greases measured at 11-BM (click for larger image).
NOTE: Consider if the grease is compatible with the temperature(s) you wish to measure. Dow Corning #4 works well for scans at room temperature and below (grease will become very viscous at higher temperatures).
If in doubt, check the grease on your lab XRD machine first.
More Information
- Wiki page on X-ray_Absorption and Fluorescence
- Chapter on X-ray absorption in the International Tables for Crystallography (2006). Vol. C, Chapter 6.3.
Please contact 11-BM staff if you have questions about sample preparation for highly absorbing materials
Examples
Visual Example
An example illustrating how (severe!) sample x-ray absorption can impact the quality of your 11-BM powder diffraction data is shown below. (click for larger image)
Numeric Examples
Example absorption calculations using Absorb web utility are shown below.
Example #1
Sample composition: C12H22O11 (Sucrose) Use default Absorb web utility values 0.41 Å, 0.4 mm, 0.6 packing fraction Chemical Formula: C12H22O11 (theoretical density ~ 1.6 g/cm3) Absorb web utility estimates μ = 0.08 cm-1 Total μ*r = 0.01
Comments: absorption is completely negligible, ignore. Note: samples with extremely low absorption may also be weakly scattering. In these cases, a larger diameter capillary may improve the counting statistics. Contact beamline staff if you have questions.
Example #2
Sample composition: Co3O4 Use default Absorb web utility values 0.41 Å, 0.4 mm, 0.6 packing fraction Chemical Formula: Co3O4 (theoretical density ~ 6.11 g/cm3) Absorb web utility estimates μ = 20.95 cm-1 (estimated density ~ 5.7 g/cm3) Total μ*r = 0.84
Comments: absorption is in normal range, μ*r less than 1.0 - no problem for this experiment
Example #3
Sample composition: BaZr0.5Ti0.5O3 Use default Absorb web utility values 0.41 Å, 0.4 mm, 0.6 packing fraction Chemical Formula: BaZr0.5Ti0.5O3 (theoretical density ~ ?? g/cm3) Absorb web utility estimates μ = 47.54 cm-1 (estimated density ~ 8.468 g/cm3) Total μ*r = 1.90
Comments: noticeable absorption, but can be used for analysis with an absorption correction term.
Example #4
Sample composition: PbZrO3 Use default Absorb web utility values 0.41 Å, 0.4 mm, 0.6 packing fraction Chemical Formula: PbZrO3 (theoretical density ~ 9.8 g/cm3) Absorb web utility estimates μ = 158.50 cm-1 (estimated density ~ 11.2 g/cm3) Total μ*r = 6.34
Comments: absorption is too large (μ*r > 5.0), alternative sample preparations are needed (silica dilution etc).
Example #5
Sample composition: Eu11Cd6Sb12 Use default Absorb web utility values 0.41 Å, 0.4 mm, 0.6 packing fraction Chemical Formula: Eu11Cd6Sb12 (theoretical density ~ 9.8 g/cm3) Absorb web utility estimates μ = 191 cm-1 (estimated density ~ 21.8 g/cm3) Total μ*r = 7.64
Comments: extreme absorption!, alternative sample preparations are needed such as dilution + smaller capillaries and/or a hollow coated capillary approach described above.