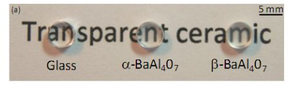Difference between revisions of "11-BM News"
| (28 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
''Periodic news and updates about 11-BM and powder diffraction at the APS'' | ''Periodic news and updates about 11-BM and powder diffraction at the APS'' | ||
== Carbon Capture Materials <small>''March 2015''</small>== | |||
Researchers use 11-BM data to make progress on new carbon capture materials. In this work published in Nature, the UC-Berkeley group of Jeff Long et al. describe their findings on a new mechanism for designing highly efficient CO2 absorbent materials. | |||
APS data from 11-BM was obtained via the beamline's unique rapid access mail-in program. High resolution powder diffraction data helped the research team solve the atomic structures of these new metal-organic frameworks materials and thus understand the mechanisms of CO2 binding. | |||
Learn more » ''[http://www.nature.com/nature/journal/vaop/ncurrent/full/nature14327.html McDonald, Long ''et al.'' Nature 2015]'' | |||
UC-Berkeley Press Release: | |||
http://newscenter.berkeley.edu/2015/03/11/new-material-captures-carbon-at-half-the-energy-cost/ | |||
<small>From the paper... Synchrotron 11-BM powder diffraction patterns and the resulting solved structures of of metal-organic frameworks. | |||
</small> | |||
[[Image:nature2015_jlong.jpg|none|300px|nature2015_jlong.jpg]] | |||
''Copyright © 2015 Nature Publishing Group'' | |||
== Morphology of NaFeSi2O6: Nanowires vs Bulk Samples <small>''Nov 2014''</small>== | |||
Researchers seeking to uncover the magnetic properties of nanowire morphology have turned to the APS 11-BM beamline. | |||
Learn more » ''[http://dx.doi.org/10.1021/ic501664x Zhou, Melot ''et al'' Inorg Chem., Publication Date (Web): November 11, 2014]'' | |||
<small>From the paper... Figure 2 shows Synchrotron diffraction pattern of products from hydrothermal preparation at temperatures ranging from 160 to 220 °C. A minimum temperature of 180 °C is required to obtain crystalline NaFeSi2O6. | |||
</small> | |||
[[Image:Merlot2014_Fig.jpg|none|200px|Merlot2014_Fig.jpg]] | |||
''Copyright © 2014 American Chemical Society'' | |||
== New high-capacity framework electrode materials for sodium-ion batteries <small>''Oct 2014''</small>== | |||
Researchers are exploring the possibilities of sodium-ion batteries by using the mail-in program at the APS 11-BM beamline. The results are published in the journal Nature Communications. The article and the 11-BM mail-in program were featured in the Argonne Newsletter in October 21. | |||
Learn more » ''[http://www.nature.com/ncomms/2014/141014/ncomms6280/abs/ncomms6280.html Lee, H.-W. ''et al'' Nat Comm 2014, Publication Date (Web): 14 October 2014]'' | |||
<small>From the paper... Figure 3 shows phase transitions and geometric considerations for Na within the subunit cell - by Synchrotron XRD patterns and schematic diagrams of structure. | |||
</small> | |||
[[Image:Lee_NatComm2014_Fig.jpeg|none|300px|Lee_NatComm2014_Fig.jpeg]] | |||
''Copyright © 2014 Macmillan Publishers Limited '' | |||
== Thermoelectric Materials optimization at 11-BM <small>''Oct 2014''</small>== | |||
Researchers using the APS 11-BM beamline have found a new method for thermoelectric optimization. The results are published in the journal Chemistry of Materials. | |||
Learn more » ''[http://dx.doi.org/10.1021/cm502588r Zevalkink et al, Chem Mater 2014, Publication Date (Web): September 23, 2014]'' | |||
<small>From the paper... Figure (A) Representative synchrotron diffraction data of the sample with nominal composition Yb0.98Zn2Sb2, including profile fit, profile difference. and profile residuals.....</small> | |||
[[Image:Zevalkink_ChemMat2014_Fig.jpeg|none|300px|Zevalkink_ChemMat2014_Fig.jpeg]] | |||
''Copyright © 2014 American Chemical Society'' | |||
== Thermoelectric materials at 11-BM <small>''Sept 2014''</small>== | |||
Researchers using beamline 11-BM at the APS are pursuing synthesis of high-purity MnSi-free single crystals. | |||
Learn more » ''[http://dx.doi.org/10.1021/cm5023823 Girard et al, Chem Mater 2014, 26, 5097−5104 ]'' | |||
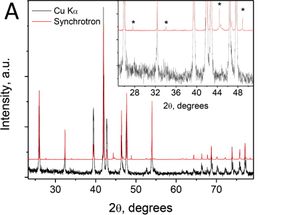
<small>From the paper... Figure (A) Demonstration of the enhanced resolution and sensitivity of the synchrotron HRPXRD of the same HMS sample made by solid-state reaction (SSR) shown in Figure 1D. The high resolution of the synchrotron HRPXRD clearly shows MnSi, whereas the laboratory Cu Kα source does not...</small> | |||
[[Image:Girard_ChemMat2014_Fig2.jpeg|none|300px|Girard_ChemMat2014_Fig2.jpeg]] | |||
''Copyright © 2014 American Chemical Society'' | |||
== Between electronic phases <small>''Sept 2014'' </small>== | |||
Researchers using the 11-BM beamline at the APS are investigating the interplay between electronic phase transitions to develop better memristors and electromagnetic modulators. Read more » | |||
Learn more » ''[http://dx.doi.org/10.1021/jp506238s Marley et al J. Phys.Chem C. 2014 118 (36), pp 21235]" | |||
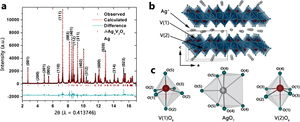
<small>From the paper... Figure 1. (a) Synchrotron powder XRD pattern (λ = 0.413 746 Å) of δ-Ag0.88V2O5 nanowires acquired at 295 K. The measured data is depicted using black circles, whereas the red lines denote the calculated diffraction pattern corresponding to the refined structure (see Table 1). The blue line plots the differential between the measured and calculated patterns. (b) The structural refinement yields a composition of δ-Ag0.88V2O5. The refined structure comprises V2O5 double layers with vanadium (red spheres) atoms coordinated to oxygen atoms in a distorted octahedral geometry (green spheres)...</small> | |||
[[Image:Marley_PhysChemC2014_Fig.jpeg|none|300px|Marley_PhysChemC2014_Fig.jpeg]] | |||
''Copyright © 2014 American Chemical Society'' | |||
== 11-BM users publish more than '''100''' papers in 2013 <small>''Jan 2014''</small>== | |||
11-BM continues to show growth in the number of publications. 2013 again saw a new record number of user publications in a year. For 2013, 11-BM was the most most productive beamline at the APS! See a publications list here: More: http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php#2013 | |||
== 11-BM Science: Progress in affordable affordable sun-powered energy <small>''Dec 2013''</small>== | == 11-BM Science: Progress in affordable affordable sun-powered energy <small>''Dec 2013''</small>== | ||
| Line 43: | Line 113: | ||
The APS X-ray Science Division is pleased to announce an enhancement to powder diffraction capabilities at the Advanced Photon Source. Effective from the 2012-3 cycle, the rapid acquisition (or ‘area detector’) powder diffraction program, formerly at beamline 1-BM, has relocated to beamline 17-BM. More Details: http://www.aps.anl.gov/Users/Communications/User_News/2012/user_news_81.html#01 | The APS X-ray Science Division is pleased to announce an enhancement to powder diffraction capabilities at the Advanced Photon Source. Effective from the 2012-3 cycle, the rapid acquisition (or ‘area detector’) powder diffraction program, formerly at beamline 1-BM, has relocated to beamline 17-BM. More Details: http://www.aps.anl.gov/Users/Communications/User_News/2012/user_news_81.html#01 | ||
A side by side comparison of APS powder diffraction beamlines is [http://11bm.xray.aps.anl.gov/documents/ | A side by side comparison of APS powder diffraction beamlines is [http://11bm.xray.aps.anl.gov/documents/APS_PowderXRD_Flyer_current.pdf here (.pdf)] | ||
==New Streamlined Process for Rapid Access Proposals <small>''Summer 2012''</small>== | ==New Streamlined Process for Rapid Access Proposals <small>''Summer 2012''</small>== | ||
Latest revision as of 12:00, 13 March 2015
Periodic news and updates about 11-BM and powder diffraction at the APS
Carbon Capture Materials March 2015
Researchers use 11-BM data to make progress on new carbon capture materials. In this work published in Nature, the UC-Berkeley group of Jeff Long et al. describe their findings on a new mechanism for designing highly efficient CO2 absorbent materials.
APS data from 11-BM was obtained via the beamline's unique rapid access mail-in program. High resolution powder diffraction data helped the research team solve the atomic structures of these new metal-organic frameworks materials and thus understand the mechanisms of CO2 binding.
Learn more » McDonald, Long et al. Nature 2015
UC-Berkeley Press Release: http://newscenter.berkeley.edu/2015/03/11/new-material-captures-carbon-at-half-the-energy-cost/
From the paper... Synchrotron 11-BM powder diffraction patterns and the resulting solved structures of of metal-organic frameworks.
Copyright © 2015 Nature Publishing Group
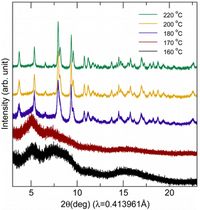
Morphology of NaFeSi2O6: Nanowires vs Bulk Samples Nov 2014
Researchers seeking to uncover the magnetic properties of nanowire morphology have turned to the APS 11-BM beamline. Learn more » Zhou, Melot et al Inorg Chem., Publication Date (Web): November 11, 2014
From the paper... Figure 2 shows Synchrotron diffraction pattern of products from hydrothermal preparation at temperatures ranging from 160 to 220 °C. A minimum temperature of 180 °C is required to obtain crystalline NaFeSi2O6.
Copyright © 2014 American Chemical Society
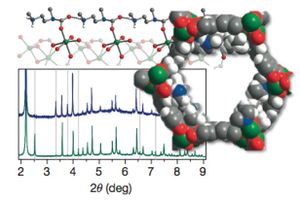
New high-capacity framework electrode materials for sodium-ion batteries Oct 2014
Researchers are exploring the possibilities of sodium-ion batteries by using the mail-in program at the APS 11-BM beamline. The results are published in the journal Nature Communications. The article and the 11-BM mail-in program were featured in the Argonne Newsletter in October 21.
Learn more » Lee, H.-W. et al Nat Comm 2014, Publication Date (Web): 14 October 2014
From the paper... Figure 3 shows phase transitions and geometric considerations for Na within the subunit cell - by Synchrotron XRD patterns and schematic diagrams of structure.
Copyright © 2014 Macmillan Publishers Limited
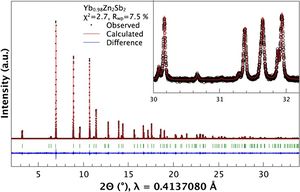
Thermoelectric Materials optimization at 11-BM Oct 2014
Researchers using the APS 11-BM beamline have found a new method for thermoelectric optimization. The results are published in the journal Chemistry of Materials.
Learn more » Zevalkink et al, Chem Mater 2014, Publication Date (Web): September 23, 2014
From the paper... Figure (A) Representative synchrotron diffraction data of the sample with nominal composition Yb0.98Zn2Sb2, including profile fit, profile difference. and profile residuals.....
Copyright © 2014 American Chemical Society
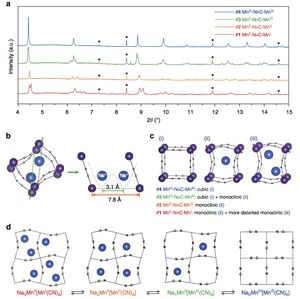
Thermoelectric materials at 11-BM Sept 2014
Researchers using beamline 11-BM at the APS are pursuing synthesis of high-purity MnSi-free single crystals.
Learn more » Girard et al, Chem Mater 2014, 26, 5097−5104
From the paper... Figure (A) Demonstration of the enhanced resolution and sensitivity of the synchrotron HRPXRD of the same HMS sample made by solid-state reaction (SSR) shown in Figure 1D. The high resolution of the synchrotron HRPXRD clearly shows MnSi, whereas the laboratory Cu Kα source does not...
Copyright © 2014 American Chemical Society
Between electronic phases Sept 2014
Researchers using the 11-BM beamline at the APS are investigating the interplay between electronic phase transitions to develop better memristors and electromagnetic modulators. Read more »
Learn more » Marley et al J. Phys.Chem C. 2014 118 (36), pp 21235"
From the paper... Figure 1. (a) Synchrotron powder XRD pattern (λ = 0.413 746 Å) of δ-Ag0.88V2O5 nanowires acquired at 295 K. The measured data is depicted using black circles, whereas the red lines denote the calculated diffraction pattern corresponding to the refined structure (see Table 1). The blue line plots the differential between the measured and calculated patterns. (b) The structural refinement yields a composition of δ-Ag0.88V2O5. The refined structure comprises V2O5 double layers with vanadium (red spheres) atoms coordinated to oxygen atoms in a distorted octahedral geometry (green spheres)...
Copyright © 2014 American Chemical Society
11-BM users publish more than 100 papers in 2013 Jan 2014
11-BM continues to show growth in the number of publications. 2013 again saw a new record number of user publications in a year. For 2013, 11-BM was the most most productive beamline at the APS! See a publications list here: More: http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php#2013
11-BM Science: Progress in affordable affordable sun-powered energy Dec 2013
An affordable sun-powered future could be closer than we think thanks to early tests on a new new ceramic material, which was developed by a team led by scientists at the University of Pennsylvania and Drexel University. The tests were conducted, in part, at the Advanced Photon Source housed at the U.S. Department of Energy’s Argonne National Laboratory. High resolution powder diffraction data from the APS beamline 11-BM was used to establish the crystal structure of the new compound.
The work is outlined in a paper “Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials” published last month in the journal Nature
Read the Argonne press release
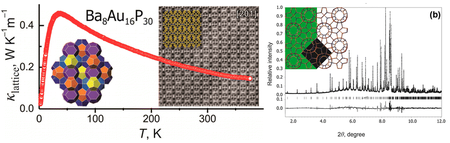
11-BM Science: New “Gold Standard” Thermoelectric Materials August 2013
A team led by chemist Dr. Kirill Kovnir of the University of California at Davis, has discovered a new class of thermoelectric materials. The group reports that Ba8Au16P30, a host-guest compound in which barium ions are trapped inside large gold-phosphorus polyhedral cages, exhibits unprecedentedly low lattice thermal conductivity. High resolution powder diffraction data from the APS beamline 11-BM was used to establish the crystal structure of the new compound.
Images Copyright © 2013 American Chemical Society
11-BM users publish more than 70 papers in 2012 April 2013
The success of 11-BM continues and is reflected by continued growth in the number of publications. In 2012 a new record was set (again!) for the total number of user publications in a year. 11-BM is now the most most productive APS-XSD division beamline, with many high impact quality publications incorporating 11-BM powder diffraction data! See a publications list here: More: http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php#2012
11-BM users make “most cited articles” list for in 2011
Congratulations! An Applied Physics Letters (APL) publication by 11-BM users from the University of Florida (Jones Group) is on the “most cited articles” list for 2011. See http://librarians.aip.org/promote/emails/aplmostcitedWV.html?TRACK=APLMC2012.
This work uses high resolution data from 11-BM to examine the previously unknown monoclinic crystal structure of polycrystalline Na0.5Bi0.5TiO3 (NBT), and important lead-free ferroelectric material for modern electronic devices.
Aksel et al, Appl. Phys. Lett. 98, 152901 (2011); http://dx.doi.org/10.1063/1.3573826 (3 pages)
New Partnership for Complementary NPD & SXPD Access at DOE User Facilities November 2012
A new partnership between Argonne (ANL) and Oak Ridge (ORNL) National Laboratories will promote complementary synchrotron & neutron diffraction science. Approved ORNL Spallation Neutron Source (SNS) users of the POWGEN neutron powder diffraction (NPD) beamline may now obtain streamlined access to high-resolution synchrotron diffraction (SXPD) data on the same samples at beamline 11-BM of the Advanced Photon Source. Because of the distinct elemental sensitivity of x-ray and neutron probes in powder diffraction, many systems benefit enormously from a complementary study exploiting both. The combined analysis of these two datasets often can reveal subtle structural details and understanding far beyond that possible with a single measurement. Contact beamline staff [email protected] or [email protected] for more details.
11-BM Science: New Highly Transparent Polycrystalline Ceramics November 2012
A recent science highlight comes from a collaboration between the APS and CNRS laboratories at Orleans. The work demonstrates a new processing route and new chemistries for highly transparent polycrystalline ceramics. These materials play an important role as optical materials for lasers, scintillators, optical lenses or transparent armor. Traditional manufacturing typically requires slow and expensive single crystal growth, and/or high temperature & high pressure processing. By contrast, the novel atomic structure of these new materials (which was characterized using high-resolution diffraction at the APS beamline 11-BM) permits low temperature processing directly from a simple glass precursor which can be easily formed into large and complex shapes.
The work was published in Advanced Materials Volume 24, Issue 41, pages 5570–5575, and was featured recently featured as a highlight on the main CNRS (French equipment of the NSF) webpage (http://www.cnrs.fr/inc/communication/direct_labos/allix.htm)
Rapid Acquisition Powder Diffraction Program Relocates to Beamline 17-BM September 2012
The APS X-ray Science Division is pleased to announce an enhancement to powder diffraction capabilities at the Advanced Photon Source. Effective from the 2012-3 cycle, the rapid acquisition (or ‘area detector’) powder diffraction program, formerly at beamline 1-BM, has relocated to beamline 17-BM. More Details: http://www.aps.anl.gov/Users/Communications/User_News/2012/user_news_81.html#01
A side by side comparison of APS powder diffraction beamlines is here (.pdf)
New Streamlined Process for Rapid Access Proposals Summer 2012
The format of the proposal for mail-in rapid access measurement requests at 11-BM has been revised. The new format features a single abstract section, and is intended to expedite and simplify the process for everyone, including users, reviewers, and staff. Rapid access requests are now reviewed by staff AND a international committee of external experts; the target review time is ~ 1 week. Read more: http://11bm.xray.aps.anl.gov/GUPguide0.html
Prolific 11-BM users publish more than 50 papers in 2011 January 2012
Thanks to the hard work of beamline staff and users, 11-BM's growing user community sets a new record for the total number of user publications in a year. 11-BM is becoming one of the most productive APS instruments, with many high impact quality publications incorporating 11-BM powder diffraction data! Learn More: http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php
Beamline Videos on 11-BM YouTube Channel Fall 2011
11-BM launches a YouTube channel. Tune in for videos highlighting the instrument, and 'how-to' videos on sample preparation and related relevant topics. See: http://www.youtube.com/user/s11bm/videos