Difference between revisions of "Air Sensitive Samples"
| (11 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
'''NOTE''' that '''ALL''' samples must fit completely inside the supplied Kapton tubes. Glass capillaries without Kapton protection, or glass capillaries that are longer than the Kapton tubes will NOT be accepted for the mail-in program and '''will be disposed''' at the beamline on receipt before any measurements. | '''NOTE''' that '''ALL''' samples must fit completely inside the supplied Kapton tubes. Glass capillaries without Kapton protection, or glass capillaries that are longer than the Kapton tubes will NOT be accepted for the mail-in program and '''will be disposed''' at the beamline on receipt before any measurements. | ||
== | == Sealed Sample Options == | ||
=== Epoxy Sealed Sample Tube === | |||
In general, we recommend the use of single or double walled epoxy sealed Kapton tubes for atmosphere sensitive samples. Most users find the Kapton tubes (especially the larger mail-in size) easier to work with inside a glove box - and the Kapton tubes will not break during the shipment to the beamline. | |||
Kapton sealed with epoxy has worked well for many users - for example with measurements of Li transition metal type air sensitive samples ([https://wiki-ext.aps.anl.gov/ug11bm/index.php/Air_Sensitive_Samples#Examples examples] are given below). These users find that epoxy sealed Kapton tubes are very air tight and can protect a sample from moisture and atmosphere contamination for many weeks. | |||
We suggest using a 5 minute epoxy to seal both ends of the supplied Kapton tube. Seal one end first; make sure to clean off epoxy on the outside of the tube before it sets. It is critical that one end of your Kapton tube still fits inside the tight Kapton tube mounting hole in the barcode labeled mounting base. After loading the powder you can seal the second end with epoxy in a glove box or bag under controlled atmosphere. | |||
These videos below (from a different beamline at the APS) demonstrate the basic concepts - '''for the sample position details you should refer to the specific 11-BM instructions that will be sent with your samples mounting bases and pre-cut Kapton capillary tubes''', | |||
Video Link | |||
*http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/Videos/LoadingSamples.html | |||
For extra protection, you are welcome to ship the assembled and prepared sample kits in a sealed plastic vial or box to reduce the chance of air exposure. But the sample will be removed from any secondary containment several hours before any measurements are performed. Do *NOT* use a glass container as the secondary containment. | |||
It is '''IMPORTANT''' to read the provided directions to find the correct position and length. If the sample is air sensitive and incorrectly prepared, we '''CAN NOT''' help to fix the sample preparation once it arrives at the facility. | |||
Below we show a photo of a large Kapton tube for the 11-BM mail-in program sealed with epoxy. Note that one end is clean - no glue outside the tube! - so that it fits inside the sample mounting base. | |||
[[Image:epoxytube.png|none|200px|epoxytube.png]] | |||
As discussed above - the use of a secondary packaging can help ensure stability of extremely sensitive samples. | |||
=== Nested Glass/Quartz Capillaries === | |||
For a guide about how to prepare glass capillaries INSIDE of the Kapton tubes, see our wiki page for [[Nesting_Glass/Quartz_Capillaries_in_Kapton_Tubes]] | |||
==Shipping Atmosphere-Sensitive Samples== | ==Shipping Atmosphere-Sensitive Samples== | ||
=== | === Secondary Containers === | ||
You can ship your atmosphere sensitive mail-in samples to the APS inside an air tight container to further protect the powders before measurements are performed. This could include a vacuum sealed bag or plastic jar. | You can ship your atmosphere sensitive mail-in samples to the APS inside an air tight container to further protect the powders before measurements are performed. This could include a vacuum sealed bag or plastic jar. | ||
| Line 35: | Line 52: | ||
== | ==Atmosphere Sensitive Sample Publications== | ||
Check the list of many [http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php 11-BM Publications] to find examples of successful atmosphere sensitive measurements performed at 11-BM by the mail-in program | Check the list of many [http://www.aps.anl.gov/Xray_Science_Division/Structural_Science/publications_11BM.php 11-BM Publications] to find examples of successful atmosphere sensitive measurements performed at 11-BM by the mail-in program; some examples are listed below: | ||
*<small>[http://dx.doi.org/10.1016/j.jfluchem.2013.12.007 Multistep synthesis of the SrFeO2F perovskite oxyfluoride via the SrFeO2 infinite-layer intermediate], Blakely, Poltavets et al. '' J. Fluorine Chem''. 159, 8 (2014).</small> | *<small>[http://dx.doi.org/10.1016/j.jfluchem.2013.12.007 Multistep synthesis of the SrFeO2F perovskite oxyfluoride via the SrFeO2 infinite-layer intermediate], Blakely, Poltavets et al. '' J. Fluorine Chem''. 159, 8 (2014).</small> | ||
*<small>[http://pubs.acs.org/doi/full/10.1021/cm4000119 Correlation Between Oxygen Vacancy, Microstrain, and Cation Distribution in Lithium-Excess Layered Oxides During the First Electrochemical Cycle,], Fell, Meng et al. ''Chem. Mater''. 25, 1621 (2013).</small> | *<small>[http://pubs.acs.org/doi/full/10.1021/cm4000119 Correlation Between Oxygen Vacancy, Microstrain, and Cation Distribution in Lithium-Excess Layered Oxides During the First Electrochemical Cycle,], Fell, Meng et al. ''Chem. Mater''. 25, 1621 (2013).</small> | ||
*<small>[http:// | *<small>[http://pubs.acs.org/doi/abs/10.1021/ja311044t Low-Potential Sodium Insertion in a NASICON-Type Structure through the Ti(III)/Ti(II) Redox Couple], Senguttuvan, Tarascon, Palacín et al. ''J. Am. Chem. Soc''. 135, 3897 (2013).</small> | ||
*<small>[http:// | *<small>[http://pubs.rsc.org/en/Content/ArticleLanding/2012/JM/c2jm16436a Degradation and (de)lithiation processes in the high capacity battery material LiFeBO3], Bo, Grey, Khalifah et al. ''J. Mater. Chem''. 22, 8799 (2012).</small> | ||
== Example: Comparing Kapton and Quartz Sealed Capillaries == | |||
===Introduction=== | |||
The examples below were very kindly provided by 11-BM mail-in users at the CNRS-LRCS laboratory in Amiens, France. In particular we say ''merci beaucoup'' to Drs. Marine Reynaud, and Josh Kurzman for providing these data and experimental details. | |||
Some of this work was published here: | |||
*<small>[http://dx.doi.org/10.1016/j.elecom.2012.04.027 Li2Fe(SO4)2 as a 3.83 V positive electrode material], Reynaud, Tarascon et al. ''Electrochemistry Communications'' 2012, 21, 77–80.</small> | |||
*<small>[http://pubs.acs.org/doi/abs/10.1021/ic401280e Marinite Li2M(SO4)2 (M = Co, Fe, Mn) and Li1Fe(SO4)2: Model Compounds for Super-Super-Exchange Magnetic Interactions], Reynaud, Tarascon et al. ''Inorganic Chemistry '' 2013, 52, 10456-10466.</small> | |||
*<small>[http://www.sciencedirect.com/science/article/pii/S1293255813002379# Brønsted acidebase reactions with anhydrous sulfamates as a pathway to [SO3N]3-containing compounds: Preparation of Li3SO3N], Kurzman et al. ''Solid State Sciences'' 2013, 25, 28-32.</small> | |||
===Comparison of the atmosphere protection=== | |||
'''Comparison of the atmosphere protection of epoxy-sealed Kapton tubes vs. flame-sealed quartz capillaries for Synchrotron X-ray diffraction measurements | |||
''' | |||
''by Marine Reynaud'' | |||
of epoxy-sealed Kapton tubes vs. flame-sealed quartz capillaries for Synchrotron X-ray diffraction measurements | |||
Marine Reynaud | |||
Collection of X-ray diffraction patterns for air- and/or moisture-sensitive samples can be an issue. Two options are currently proposed for the preparation of such samples for their measurement via the 11-BM mail-in service. The first one is to seal the sample into a Kapton tube using epoxy glue; however the gas permeability of Kapton was not really known up to now. Alternatively, the sensitive powders can be sealed in a glass or quartz capillary, prior being nested in the Kapton tube; however these capillaries are more likely to be broken during the shipment to the beamline, and the quality of the data may be reduced by the use of the extra glass or quartz capillary. | Collection of X-ray diffraction patterns for air- and/or moisture-sensitive samples can be an issue. Two options are currently proposed for the preparation of such samples for their measurement via the 11-BM mail-in service. The first one is to seal the sample into a Kapton tube using epoxy glue; however the gas permeability of Kapton was not really known up to now. Alternatively, the sensitive powders can be sealed in a glass or quartz capillary, prior being nested in the Kapton tube; however these capillaries are more likely to be broken during the shipment to the beamline, and the quality of the data may be reduced by the use of the extra glass or quartz capillary. | ||
Therefore, the aim of the present study was to evaluate the effectiveness of the atmosphere protection of epoxy-sealed Kapton tubes compared to flame-sealed quartz capillaries. Two samples of different degree of air/moisture-sensitivity were measured in both conditions (epoxy-sealed kapton tube and flame-sealed quartz capillaries) through the 11-BM mail-in service. | Therefore, the aim of the present study was to evaluate the effectiveness of the atmosphere protection of epoxy-sealed Kapton tubes compared to flame-sealed quartz capillaries. Two samples of different degree of air/moisture-sensitivity were measured in both conditions (epoxy-sealed kapton tube and flame-sealed quartz capillaries) through the 11-BM mail-in service. | ||
===Samples | ===Samples Preparation Details=== | ||
Epoxy-sealed Kapton tubes: | *'''Epoxy-sealed Kapton tubes:''' | ||
1.5-mm diameter Kapton tubes were first fixed to the sample base using one drop of epoxy glue in the open air. Then the powder samples were filled into the kapton tubes inside an argon-filled glove- box, and the tubes were sealed with another drop of epoxy glue. Note that the epoxy glue takes more time to dry under argon than under air, and at least one night was needed for the glue to harden in the glove-box. For the shipment, the bases were then packed into a coffee-bag sealed under argon, which was opened by the 11-BM staff only few hours before the XRD measurement. | 1.5-mm diameter Kapton tubes were first fixed to the sample base using one drop of epoxy glue in the open air. Then the powder samples were filled into the kapton tubes inside an argon-filled glove- box, and the tubes were sealed with another drop of epoxy glue. Note that the epoxy glue takes more time to dry under argon than under air, and at least one night was needed for the glue to harden in the glove-box. For the shipment, the bases were then packed into a coffee-bag sealed under argon, which was opened by the 11-BM staff only few hours before the XRD measurement. | ||
Flame-sealed quartz capillaries: | *'''Flame-sealed quartz capillaries''': | ||
0.7-mm diameter quartz capillaries were filled with the powder samples into an argon-filled glove- box. After being plugged with a drop of epoxy-glue under argon, the capillaries were brought out of the glove-box and immediately sealed with the flame of a lighter. Then, the as-sealed capillaries were fitted into 0.8-mm diameter kapton tubes and securely fixed with clay or glue. Note that in this case, the sample bases were packed under air to be sent to the 11-BM beamline. | 0.7-mm diameter quartz capillaries were filled with the powder samples into an argon-filled glove- box. After being plugged with a drop of epoxy-glue under argon, the capillaries were brought out of the glove-box and immediately sealed with the flame of a lighter. Then, the as-sealed capillaries were fitted into 0.8-mm diameter kapton tubes and securely fixed with clay or glue. Note that in this case, the sample bases were packed under air to be sent to the 11-BM beamline. | ||
*Results | |||
====Sample 1: LiFe(SO4)2 ==== | ====Sample 1: LiFe(SO4)2 ==== | ||
LiFe(SO4)2 is not highly air-sensitive, but it does catch water if exposed few hours to air. The hydrated sample would then present a completely different XRD pattern from the anhydrous phase. | LiFe(SO4)2 is not highly air-sensitive, but it does catch water if exposed few hours to air. The hydrated sample would then present a completely different XRD pattern from the anhydrous phase. | ||
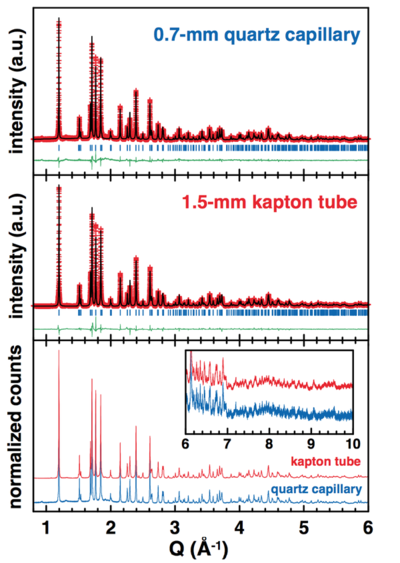
As seen from the refinement of the XRD patterns (figure below), both quartz capillary and Kapton tube have efficiently protected the LiFe(SO4)2 sample from moisture. | As seen from the refinement of the XRD patterns (figure below), both quartz capillary and Kapton tube have efficiently protected the LiFe(SO4)2 sample from moisture. | ||
However, one should note that the statistics is definitely improved when using the wider kapton tube (1.5-mm vs. 0.8 mm). | However, one should note that the statistics is definitely improved when using the wider kapton tube (1.5-mm vs. 0.8 mm). | ||
[[Image:LiFeSO42.png|none| | [[Image:LiFeSO42.png|none|400px|LiFeSO42.png]] | ||
===Sample 2: Na3SO3N === | ====Sample 2: Na3SO3N ==== | ||
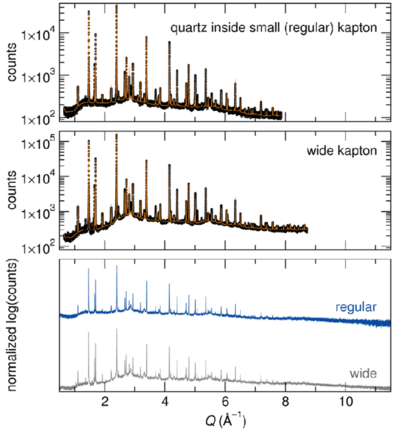
Na3SO3N is much more sensitive than LiFe(SO4)2, as it decomposes within a few minutes when exposed to air. However, despite the highly air-sensitive nature of this material, there is no evidence of decomposition in the diffraction pattern collected for the Na3SO3N sample sealed in the wide Kapton tube. | Na3SO3N is much more sensitive than LiFe(SO4)2, as it decomposes within a few minutes when exposed to air. However, despite the highly air-sensitive nature of this material, there is no evidence of decomposition in the diffraction pattern collected for the Na3SO3N sample sealed in the wide Kapton tube. | ||
As seen from a comparison of the top and middle panels in the figure presented below, the counting statistics are certainly improved by use of the wider tube; which enabled a slightly larger Q range to be refined. | As seen from a comparison of the top and middle panels in the figure presented below, the counting statistics are certainly improved by use of the wider tube; which enabled a slightly larger Q range to be refined. | ||
Note that, although the different contributions were not separated here, these are 3-phase refinements, and there is evidence for other minor impurities. The primary phase is highly disordered, and the thermal parameters are exceptionally large on all of the atom positions, particularly for those not on special positions, although they agree well with the values reported for comparable Li3PO4 refinements. Therefore, advantages of the wider capillary are perhaps obviated by the intrinsic disorder of the compound. | Note that, although the different contributions were not separated here, these are 3-phase refinements, and there is evidence for other minor impurities. The primary phase is highly disordered, and the thermal parameters are exceptionally large on all of the atom positions, particularly for those not on special positions, although they agree well with the values reported for comparable Li3PO4 refinements. Therefore, advantages of the wider capillary are perhaps obviated by the intrinsic disorder of the compound. | ||
[[Image:Na3SO3N.png|none| | [[Image:Na3SO3N.png|none|400px|Na3SO3N.png]] | ||
===Conclusion === | ===User Conclusion=== | ||
In conclusion, both epoxy-sealed Kapton tubes and flame-sealed quartz capillaries similarly preserved the samples from air exposure. | In conclusion, both epoxy-sealed Kapton tubes and flame-sealed quartz capillaries similarly preserved the samples from air exposure. | ||
A noticeable advantage of using epoxy-sealed Kapton tubes is that the entire sample preparation can be carried out in the glove-box, whereas sealing the quartz capillaries with a flame presents the risk of oxidizing the sample. Moreover, Kapton tubes are much less likely to be broken during the shipment or the manipulation of the sample compared to quartz capillaries. | A noticeable advantage of using epoxy-sealed Kapton tubes is that the entire sample preparation can be carried out in the glove-box, whereas sealing the quartz capillaries with a flame presents the risk of oxidizing the sample. Moreover, Kapton tubes are much less likely to be broken during the shipment or the manipulation of the sample compared to quartz capillaries.. | ||
Latest revision as of 13:37, 23 October 2014
Information for 11-BM users who have mail-in samples that are moisture or atmosphere sensitive.
Introduction
Many mail-in users for powder diffraction at 11-BM have successfully sent and measured atmosphere sensitive samples. Most users send either 1) samples with powders directly sealed inside the Kapton capillary tubes supplied by the beamline using epoxy on the tubes ends, or 2) samples sealed inside extra glass or quartz sealed capillaries which are then nested inside the Kapton tubes before sending to the beamline.
Both methods could be used with sample powders prepared and loaded inside a glove box.
NOTE that ALL samples must fit completely inside the supplied Kapton tubes. Glass capillaries without Kapton protection, or glass capillaries that are longer than the Kapton tubes will NOT be accepted for the mail-in program and will be disposed at the beamline on receipt before any measurements.
Sealed Sample Options
Epoxy Sealed Sample Tube
In general, we recommend the use of single or double walled epoxy sealed Kapton tubes for atmosphere sensitive samples. Most users find the Kapton tubes (especially the larger mail-in size) easier to work with inside a glove box - and the Kapton tubes will not break during the shipment to the beamline.
Kapton sealed with epoxy has worked well for many users - for example with measurements of Li transition metal type air sensitive samples (examples are given below). These users find that epoxy sealed Kapton tubes are very air tight and can protect a sample from moisture and atmosphere contamination for many weeks.
We suggest using a 5 minute epoxy to seal both ends of the supplied Kapton tube. Seal one end first; make sure to clean off epoxy on the outside of the tube before it sets. It is critical that one end of your Kapton tube still fits inside the tight Kapton tube mounting hole in the barcode labeled mounting base. After loading the powder you can seal the second end with epoxy in a glove box or bag under controlled atmosphere.
These videos below (from a different beamline at the APS) demonstrate the basic concepts - for the sample position details you should refer to the specific 11-BM instructions that will be sent with your samples mounting bases and pre-cut Kapton capillary tubes,
Video Link
For extra protection, you are welcome to ship the assembled and prepared sample kits in a sealed plastic vial or box to reduce the chance of air exposure. But the sample will be removed from any secondary containment several hours before any measurements are performed. Do *NOT* use a glass container as the secondary containment.
It is IMPORTANT to read the provided directions to find the correct position and length. If the sample is air sensitive and incorrectly prepared, we CAN NOT help to fix the sample preparation once it arrives at the facility.
Below we show a photo of a large Kapton tube for the 11-BM mail-in program sealed with epoxy. Note that one end is clean - no glue outside the tube! - so that it fits inside the sample mounting base.
As discussed above - the use of a secondary packaging can help ensure stability of extremely sensitive samples.
Nested Glass/Quartz Capillaries
For a guide about how to prepare glass capillaries INSIDE of the Kapton tubes, see our wiki page for Nesting_Glass/Quartz_Capillaries_in_Kapton_Tubes
Shipping Atmosphere-Sensitive Samples
Secondary Containers
You can ship your atmosphere sensitive mail-in samples to the APS inside an air tight container to further protect the powders before measurements are performed. This could include a vacuum sealed bag or plastic jar.
Include a note in your shipment package - with your name, the proposal number and included sample base numbers - to request that any sealed extra containers for samples NOT be opened by beamline staff immediately. If you request with a note - we can keep your samples inside the extra containers until ~ 24 hours before the measurements are performed.
NOTE: NO extra GLASS! The use of glass vials, ampoules or other containers for shipping 11-BM samples to the APS is now prohibited. (excluding nested sample quartz capillaries). Instead, simply use bubble wrap or plastic containers. Most of the time, glass containers are broken on arrival at the APS, which is dangerous for beamline staff and does nothing to protect your samples
Measurement Calendar
Mail-in samples at 11-BM are queued for measurement in the order they arrive at the beamline. It is NOT possible to schedule a exact measurement time or date for your samples.
However, if you contact beamline staff - we give some advice about which times might be good (or bad!) to send atmosphere sensitive samples, in order to reduce the time the samples spend queued waiting for a measurement.
Be aware that the APS and beamline 11-BM is shutdown three times per year for maintenance. Typically it is the months of January, May, and September. Check the APS calendar for specific dates. You might wish to avoid sending atmosphere sensitive samples just before or during these shutdown periods.
Atmosphere Sensitive Sample Publications
Check the list of many 11-BM Publications to find examples of successful atmosphere sensitive measurements performed at 11-BM by the mail-in program; some examples are listed below:
- Multistep synthesis of the SrFeO2F perovskite oxyfluoride via the SrFeO2 infinite-layer intermediate, Blakely, Poltavets et al. J. Fluorine Chem. 159, 8 (2014).
- Correlation Between Oxygen Vacancy, Microstrain, and Cation Distribution in Lithium-Excess Layered Oxides During the First Electrochemical Cycle,, Fell, Meng et al. Chem. Mater. 25, 1621 (2013).
- Low-Potential Sodium Insertion in a NASICON-Type Structure through the Ti(III)/Ti(II) Redox Couple, Senguttuvan, Tarascon, Palacín et al. J. Am. Chem. Soc. 135, 3897 (2013).
- Degradation and (de)lithiation processes in the high capacity battery material LiFeBO3, Bo, Grey, Khalifah et al. J. Mater. Chem. 22, 8799 (2012).
Example: Comparing Kapton and Quartz Sealed Capillaries
Introduction
The examples below were very kindly provided by 11-BM mail-in users at the CNRS-LRCS laboratory in Amiens, France. In particular we say merci beaucoup to Drs. Marine Reynaud, and Josh Kurzman for providing these data and experimental details.
Some of this work was published here:
- Li2Fe(SO4)2 as a 3.83 V positive electrode material, Reynaud, Tarascon et al. Electrochemistry Communications 2012, 21, 77–80.
- Marinite Li2M(SO4)2 (M = Co, Fe, Mn) and Li1Fe(SO4)2: Model Compounds for Super-Super-Exchange Magnetic Interactions, Reynaud, Tarascon et al. Inorganic Chemistry 2013, 52, 10456-10466.
- Brønsted acidebase reactions with anhydrous sulfamates as a pathway to [SO3N3-containing compounds: Preparation of Li3SO3N], Kurzman et al. Solid State Sciences 2013, 25, 28-32.
Comparison of the atmosphere protection
Comparison of the atmosphere protection of epoxy-sealed Kapton tubes vs. flame-sealed quartz capillaries for Synchrotron X-ray diffraction measurements by Marine Reynaud
Collection of X-ray diffraction patterns for air- and/or moisture-sensitive samples can be an issue. Two options are currently proposed for the preparation of such samples for their measurement via the 11-BM mail-in service. The first one is to seal the sample into a Kapton tube using epoxy glue; however the gas permeability of Kapton was not really known up to now. Alternatively, the sensitive powders can be sealed in a glass or quartz capillary, prior being nested in the Kapton tube; however these capillaries are more likely to be broken during the shipment to the beamline, and the quality of the data may be reduced by the use of the extra glass or quartz capillary. Therefore, the aim of the present study was to evaluate the effectiveness of the atmosphere protection of epoxy-sealed Kapton tubes compared to flame-sealed quartz capillaries. Two samples of different degree of air/moisture-sensitivity were measured in both conditions (epoxy-sealed kapton tube and flame-sealed quartz capillaries) through the 11-BM mail-in service.
Samples Preparation Details
- Epoxy-sealed Kapton tubes:
1.5-mm diameter Kapton tubes were first fixed to the sample base using one drop of epoxy glue in the open air. Then the powder samples were filled into the kapton tubes inside an argon-filled glove- box, and the tubes were sealed with another drop of epoxy glue. Note that the epoxy glue takes more time to dry under argon than under air, and at least one night was needed for the glue to harden in the glove-box. For the shipment, the bases were then packed into a coffee-bag sealed under argon, which was opened by the 11-BM staff only few hours before the XRD measurement.
- Flame-sealed quartz capillaries:
0.7-mm diameter quartz capillaries were filled with the powder samples into an argon-filled glove- box. After being plugged with a drop of epoxy-glue under argon, the capillaries were brought out of the glove-box and immediately sealed with the flame of a lighter. Then, the as-sealed capillaries were fitted into 0.8-mm diameter kapton tubes and securely fixed with clay or glue. Note that in this case, the sample bases were packed under air to be sent to the 11-BM beamline.
- Results
Sample 1: LiFe(SO4)2
LiFe(SO4)2 is not highly air-sensitive, but it does catch water if exposed few hours to air. The hydrated sample would then present a completely different XRD pattern from the anhydrous phase. As seen from the refinement of the XRD patterns (figure below), both quartz capillary and Kapton tube have efficiently protected the LiFe(SO4)2 sample from moisture. However, one should note that the statistics is definitely improved when using the wider kapton tube (1.5-mm vs. 0.8 mm).
Sample 2: Na3SO3N
Na3SO3N is much more sensitive than LiFe(SO4)2, as it decomposes within a few minutes when exposed to air. However, despite the highly air-sensitive nature of this material, there is no evidence of decomposition in the diffraction pattern collected for the Na3SO3N sample sealed in the wide Kapton tube. As seen from a comparison of the top and middle panels in the figure presented below, the counting statistics are certainly improved by use of the wider tube; which enabled a slightly larger Q range to be refined. Note that, although the different contributions were not separated here, these are 3-phase refinements, and there is evidence for other minor impurities. The primary phase is highly disordered, and the thermal parameters are exceptionally large on all of the atom positions, particularly for those not on special positions, although they agree well with the values reported for comparable Li3PO4 refinements. Therefore, advantages of the wider capillary are perhaps obviated by the intrinsic disorder of the compound.
User Conclusion
In conclusion, both epoxy-sealed Kapton tubes and flame-sealed quartz capillaries similarly preserved the samples from air exposure. A noticeable advantage of using epoxy-sealed Kapton tubes is that the entire sample preparation can be carried out in the glove-box, whereas sealing the quartz capillaries with a flame presents the risk of oxidizing the sample. Moreover, Kapton tubes are much less likely to be broken during the shipment or the manipulation of the sample compared to quartz capillaries..